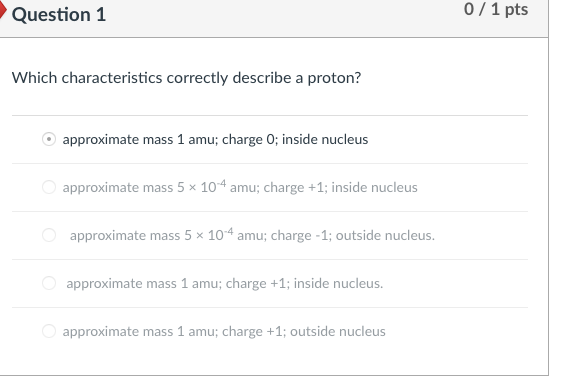
Which Characteristics Correctly Describe a Proton
Inside nucleus B approximate mass 5 10-4 amu. Relative mass of 1.

States Of Matter Doodle Notes Solid Liquid Gas Plasma Phase Changes Video Video Doodle Notes Doodles Chemistry Lessons
1 The smallest amount of an element that retains that elements characteristics is the.

. Which characteristics correctly describe a protonAapproximate mass 5 10-4 amu. A approximate mass 1 amu. Approximate mass 1 amu.
These two isotopes are designated by the symbols 113 49 In and 115 49 In. 9 The value of Z for an atom containing 47 protons 47 electrons and 60 neutrons is. Inside nucleus 11 An atom containing 29 protons 29 electrons.
2 Another name for atomic mass unit amu is the. Which characteristics correctly describe a proton. C converts it to an isotope of the same element.
5 rows Which characteristics correctly describe a proton. Inside nucleus approximate mass 5 10-4 amu. Which characteristics correctly describe a proton.
A approximate mass 5 10-4 amu. Relative mass of 1. Which characteristics correctly describe a proton.
The smallest amount of an element that retains that elementʹs characteristics is the. 3 Which characteristics correctly describe a proton. Uranium-238 absorbs a neutron and then emits a beta particle.
Proton stable subatomic particle that has a positive charge equal in magnitude to a unit of electron charge and a rest mass of 167262 10 27 kg which is. D converts it to an atom of a different element. Approximate mass 1 amu.
The plum pudding model of the atom was. 8 An atom containing 47 protons 47 electrons and 60 neutrons has a mass number of A 13 B 47 C 60 D 107 E 154. Inside nucleus approximate mass 1 amu.
Inside nucleus B approximate mass 5 10-4 amu. A approximate mass 1 amu. Isotope Abundance Mass amu 3060 15937 sig 1579 16279 X 5361 1 6392 a.
Neutrons have the ability to deeply penetrate matter they interact with nuclei and neutrons unlike X-rays can. Inside nucleus Which particle has a mass approximately equal to the mass of a proton. - Answers They have mass electrical charge positive use the strong nuclear force to bond with neutrons to form atomic nucleui they have energy have.
A approximate mass 1 amu. The element indium consists of two isotopes. Which particle has a mass approximately equal to the mass of a proton.
Outside nucleus Bapproximate mass 1 amu. 26 Adding one proton to the nucleus of an atom A increases its atomic mass by one unit but does not change its atomic number. Inside nucleus Capproximate mass 1 amu.
E increases its atomic number by one unit but does not change its atomic mass. B does not change either its atomic number or its atomic mass. Neutrons are electrically neutral but have a spin or magnetic moment so they they are sensitive to magnetic sources in condensed matter and can provide images of magnetic structure.
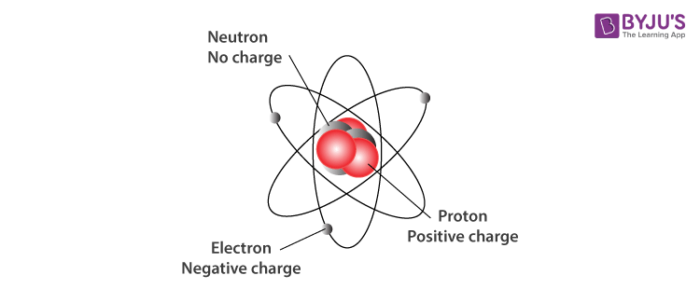
3 Which characteristics correctly describe a proton. A proton is one of three main particles that make up the atom. Protons have a positive electrical charge of one 1 and a mass of 1 atomic mass unit amu which is about 167 10 27 kilograms.
Another name for atomic mass unit amu is the. 10 Which characteristics correctly describe a proton. 3 Which characteristics correctly describe a proton.
Protons are found in the nucleus of the atom. What are the characteristics of protons. Which characteristics correctly describe a proton.
Charge 1. Combining a neutron with a proton makes the proton electrically neutral. 10 The value of Z for the element Ar is A 37 B 39945 C 19 D 21945 E 18.
Relative mass of 5 Ã 10-4. How many neutrons are in the nucleus of an atom that has an atomic number of 17 and a mass number of 357 a. Approximate mass 1 amu.
Show that the resulting nucleus is neptunium-239. Relative mass of 1. The average atomic mass of the element amu.
Relative mass of 1. Approximate mass 5 x 10 -4 amu. Which characteristics correctly describe a proton.
Approximate mass 5 10-4 amu. Inside nucleus 4 Which particle has a mass approximately equal to the mass of a proton. Outside nucleus approximate mass 1 amu.
Outside nucleus approximate mass 1 amu. Which characteristics correctly describe a proton. Relative mass of 5 Ã 10-4.
Approximate mass 5 x 10 -4 amu. The characteristics of neutrons. This is a tiny dense region at the center of the atom.

How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative
2 1 Electrons Protons Neutrons And Atoms Physical Geology
Solved 0 1 Pts Question 1 Which Characteristics Correctly Chegg Com
Subatomic Particles Definition Discovery And Key Features

Compare The Properties Of Protons Electrons And Neutrons

Discovery Of Proton And Neutron Who Discovered And How Proton And Neutron Were Discovered With Faqs And Images

Bright Ideas Speech Language Pathology Outer Space Theme Speech And Language Speech Therapy Themes Space Theme

States Of Matter Doodle Notes Solid Liquid Gas Plasma Phase Changes Video Video Doodle Notes Doodles Chemistry Lessons

Create A Concept Map Of Biomolecules Concept Map Biology Activity Graphic Organizers

Mass Of Proton Definition Charge Discovery Properties With Videos

Introduction To Structure Of Atom Proton Neutron Electron With Examples
Chemistry I Atoms And Molecules

Atomic Structure Electrons Protons Neutrons And Atomic Models

Introduction To Structure Of Atom Proton Neutron Electron With Examples

Labeled Parts Of An Atom Diagram Atom Diagram Atom Worksheets

The Most Beautiful Chemistry Videos I Ve Ever Seen Chemistry Lessons Chemistry Science Chemistry


Comments
Post a Comment